- Europe
- Americas
- Asia and Middle East
- Africa and Oceania
June 10 - 14, 2024 | Frankfurt am Main, Germany
ACHEMA - Inspiring Sustainable Connections
MOVE on with your automation upgrade
At Booth J72 in Hall 3.1 you will experience how specialized robots and mobile robots support the evolution of the pharmaceutical industry and help manufacturers adapt in this transformational era.
Stricter regulations, notably the EU’s revised GMP Annex 1 and the FDA’s updated cGMP standards, pose new technical and commercial challenges. Annex 1 emphasizes the need to reduce human intervention in aseptic processes and increase automation with robotics and other technologies to avoid contamination while scaling production. Mobile solutions such as AGV platforms are an especially promising area of development in pharmaceutical manufacturing and compliance.
SPECIAL EVENT: Pharma Impulses X ACHEMA 2024
Welcome to our LIVE stream at our ACHEMA booth starting at 10.30 am. Don’t miss it out in person or join online.
-

Automation Upgrade
What matters for pharmaceutical manufacturing are cleanability, compatibility with decontamination media and processes, and particle emissions as defined by cleanroom classifications. Discover more -

Pharma Offering
Changing market demands and stricter regulations define the current pharmaceutical industry landscape. Stäubli Robotics has validated solutions: dedicated industrial robots for each pharma grade environment. Discover more -
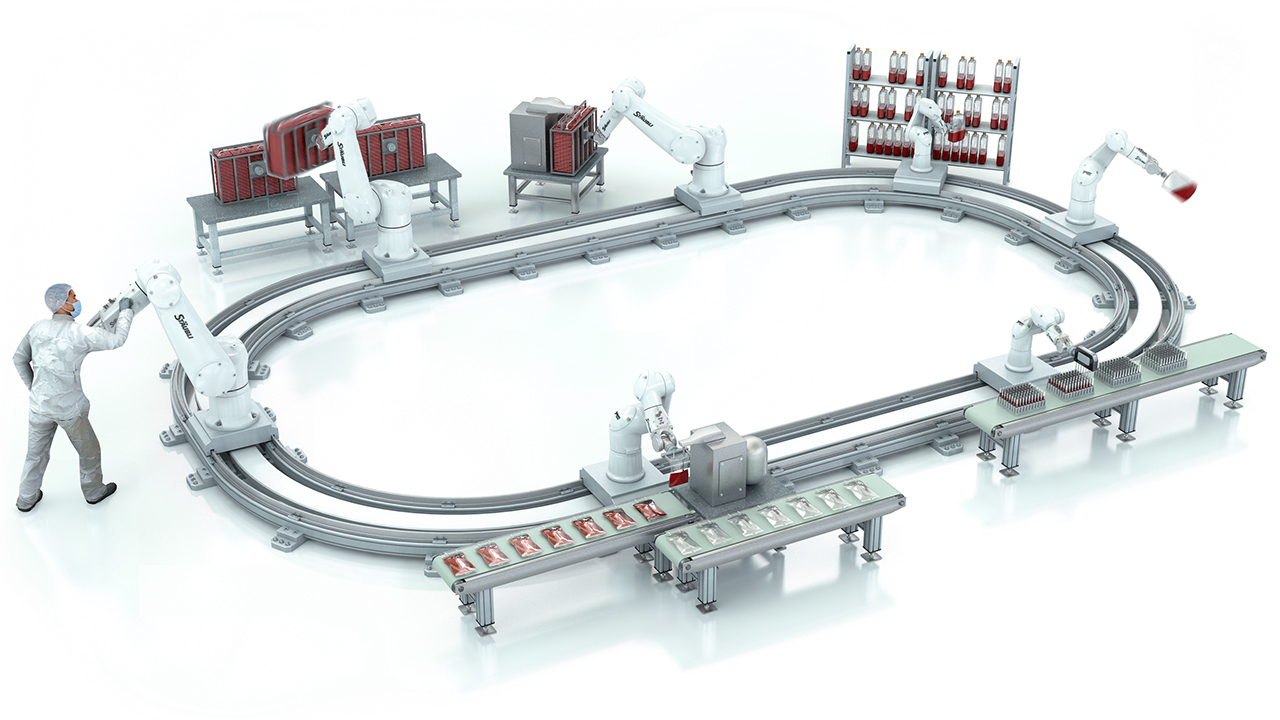
Biotherapy
Automating biotherapy production at the speed of market expansion. Suppliers and system integrators are finding new ways to transform production, from Grade A/B environments to inspection, final packaging and palettizing. Discover more -

Newsroom
Check out our ACHEMA Newsroom and find press releases about Stäubli robots in pharma industry
Stäubli Partner at ACHEMA 2024
- Teaser
- Teaser
