Drug production
Liquid and solid production
Pharmaceutical manufacturing is a highly controlled and regulated environment. The production is made in an isolator, where decontamination is mandatory and monitored. Almost every process can be automated to gain in flexibility and sterility.
Get inspired by our solutions from API production to end of line packaging:
The Active Pharmaceutical Ingredient industry (API) are manufactured from raw materials through both chemical and physical means. Depending on the complexity of the molecule required, synthesis of APIs might need multi-step complex chemistry utilizing a range of processing technologies. Regardless of where the active pharmaceutical ingredient is made, companies must adhere to strict safety and quality standards set by the country where it will be used.




Small Batch API production within Isolator with TX2-160 Stericlean opening powder bulk and feeding filling line

Example of Mobility upgrade where TX2-160 unload empty bulk from Isolator and use AGV for transportation to logistic area
Main applications
- Bulk powder handling
- Transportation of API Vessel
- API logistics
❝ Robots become the ideal partner to handle sensitive substances, allowing people to devote themselves to tasks where their knowledge is irreplaceable. ❞
RUDOLF MICHAEL WEISS | GLOBAL HEAD OF PHARMA ROBOTICS
Once individually packed, the journey to shipment starts. The requirements within the secondary packaging area is less restrictive than within an isolator, however the cleanability of equipment still has an essential role. Throughout the task, traceability is mandatory, with robotics offering huge flexibility for inspection at each stage of the process.
With quality assurance having the most stringent requirements, we foresee more and more demand on inspection units. On the secondary packaging area, collaborative aspects of the robots can be considered to ensure safety of the workers while sustaining high levels of productivity.





TX2-60 places a filled syringe into a blister

SCARA robot for secondary packaging

Palletizing with TX2-160 and Automated forklift move the pallet to Logistic area
Main applications
- Container Handling
- Inspection
- Device and component assembly
- Blistering
- Labelling
- Cartoning
- Transferring
- Palettizing
- Tracking
❝ From the first molecule to the shipment, robots play an essential role in the pharma manufacturing chain. ❞
RUDOLF MICHAEL WEISS | GLOBAL HEAD OF PHARMA ROBOTICS
Success stories
-

Pharmaceutical packaging
Secondary packaging of parenteral drugs requires a great deal of automation expertise. Uhlmann Pac-Systeme knows how it works and relies on highly dynamic four-axis robots from Stäubli in its UPS 5 blister machine. -

Assembly and packaging of cryogas cartridges
Two Stäubli robots, a SCARA and a six-axis machine, exemplify the contribution that automation can make to efficiency in the assembly and packaging of gas cartridges for CryoPens, the new wonder tool for the removal of skin blemishes. -

Drug inspection
A new testing technology has ushered in a new chapter in the inspection of pharmaceutical products. The innovative robotic cell uses a Stäubli TX40 compact robotic arm to facilitate automatic inspection of small batches. -
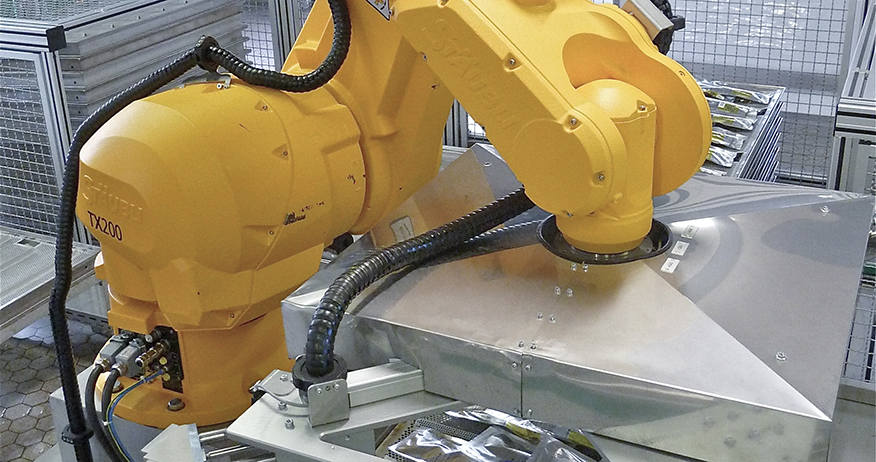
Automatic packaging of IV bags
Bayer Shering Pharma uses two robotic arms from Stäubli to automatically fill and package its IV bags. The RX160 and TX200 robots provide continuous production in a cleanroom environment.








